Reusable Injection Device
As part of my master thesis in industrial design engineering, I collaborated with SHL Medical and co-wrote together with Hala Mawas. The aim of the thesis was to deliver an in-depth understanding of user preferences for disposable and reusable injection devices from a sustainability perspective, provide concepts for future device designs, and contribute to the ongoing discussion around reducing medical device waste while enhancing user satisfaction.
The problem
The growing interest in, and demand for, the development of sustainable injection devices has increased pressure on companies to reassess their products and explore opportunities for improvement. As single use autoinjectors generate a significant amount of plastic waste, growing concerns about sustainability has led companies to further explore reusable autoinjectors to reduce and minimize the environmental impact. At the same time, the preferences for single use versus reusable systems, and the potential for making current autoinjectors more sustainable, remain insufficiently explored.
Transitioning towards more sustainable practices in the medical sector requires overcoming considerable field-specific challenges linked to strict hygiene, safety and usability requirements. One aspect of this is regulatory requirements - as an example, autoinjectors must adhere to applicable parts of the product standard series of ISO 11608. Another aspect is user needs and expectations. Even though disposable single-use injection devices generate massive waste, users are accustomed to their convenience and reliability - alternatives might not gain traction if using them is time-consuming, if they are overly costly, or if they cannot provide the same guarantees of proper drug administration. To reduce waste and ecological harm in a way that scales, reusable IDs that overcome these challenges are needed.
In order to develop an environmentally viable device that is in line with usability requirements while being accepted or even cherished by users, there is great benefit in reexploring injection device design. In this process, care must be taken to understand user perceptions of new potential device features in comparison to devices users are already familiar with.
The prototype
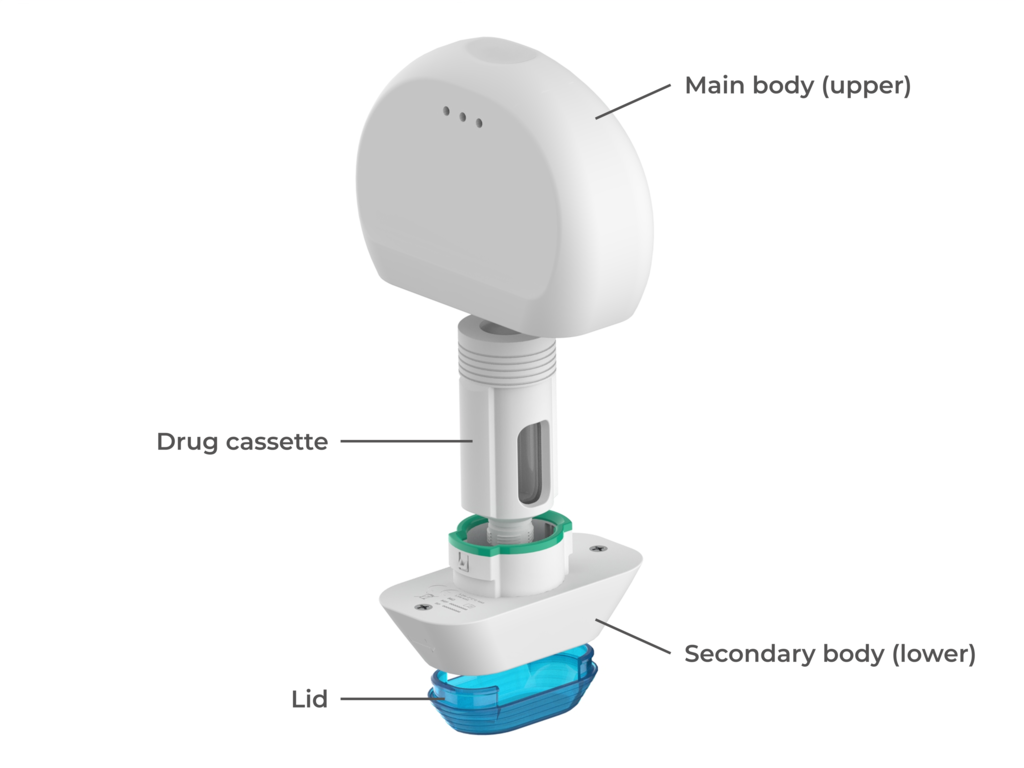
Minnie is a versatile electromechanical injection device that can deliver variable doses through a reusable power unit. Minnie utilises replaceable drug containers, called cassettes, containing a pre-filled cartridge to deliver medication. Minnie must be used with an accompanying app and is part of a take-back program for both the cassettes and the device itself.
The main dimensions of Minnie are approximately 75×70×30 mm making it a compact and easily portable device that fits in the bag. The total weight is approximately 120 g, which contributes to its lightweight and ease of handling during everyday use.
The overall aesthetic incorporates smooth curves around the injection button and the finger rest, evoking a sense of kindness that aligns with the SHL brand identity. The rounded outer edges further enhance ergonomics, offering a relaxed grip that allows the user to hold the device comfortably and naturally during operation. Additionally, the matte texture communicates reliability and conveys quality, while offering enough friction to allow a good grip of the device.
Minnie should be used together with standard needles which are handled and attached according to manufacturer instructions. The use of standard needles gives users the freedom to choose the needle best suited for their needs.
The final concept is made up of four subsystems:
- The main device body (upper)
- The secondary device body (lower)
- The drug cassette, housing a 2.1 ml cartridge
- The lid
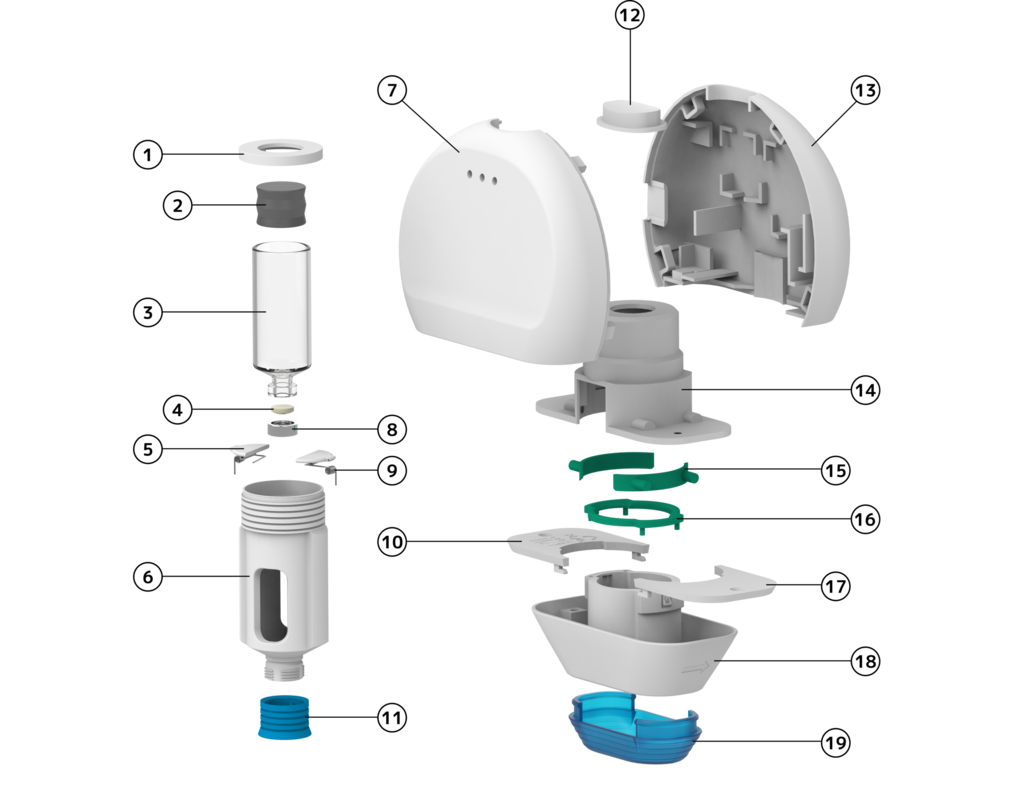
The main (upper) body contains the electronics (i.e. motor, linear actuator, battery, circuit board, RAM, LED lights, Bluetooth module, USB-C port, RFID reader and IR sensor) and houses the bayonet fitting for the attachment of the secondary body. The device only has one button, used to initiate different functions, located at the top of the main body: A double-click turns the device on or off, while a long click initiates the injection.
The area on the secondary (lower) body directly in contact with the main body is used to display the CE marking and other manufacturing information, while the lower front or back of the device can be used to display branding. The placement of this information can also be placed on the main body (mirroring the placement) depending on the desired visibility of the information. The drug labelling is only available on the cassette and contains a QR-code for additional information about the drug or instructions for use.
The main dimensions of the cassette are 50x20x22 mm, which makes it capable of housing a 2.1 ml vial. When the cassette is properly inserted, it is completely covered inside the device. To change the cassette, the secondary body is used as a tool to provide better grip and unfasten the cassette after use: turning the secondary body by 90° unhooks the bayonet fitting and detaches the cassette from the main body. The cassette is then simply pulled from the secondary body and can be discarded. The reverse process is used to insert a new cassette.
The lid is designed to protect against dirt and debris entering the device and contaminating the cartridge septum. The outer surface is detailed with ridges to enhance the grip when removing the lid. The transparent material enables users to visually check if a cassette is still inserted after use.
A sample label that mimics Elexy’s labelling was created for the cassette to showcase how the SHL brand can be reflected in Minnie. The label is the main communication channel on the device itself, and any necessary information about the medication should be displayed here (e.g. expiration date, storage conditions, logos, drug amount, QR code, lot number).
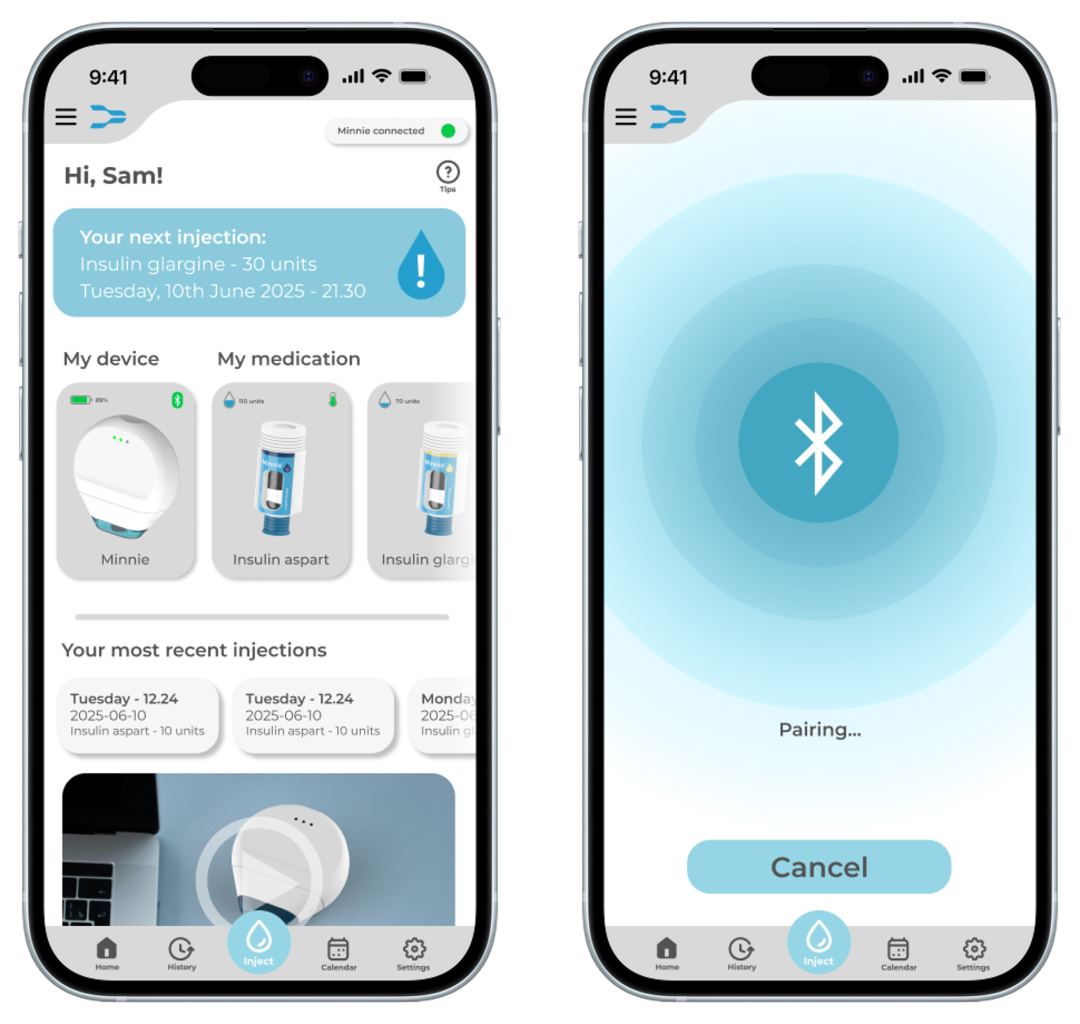
The necessary accompaniments for the functionality of Minnie are the accompanying app as well as a take-back program.
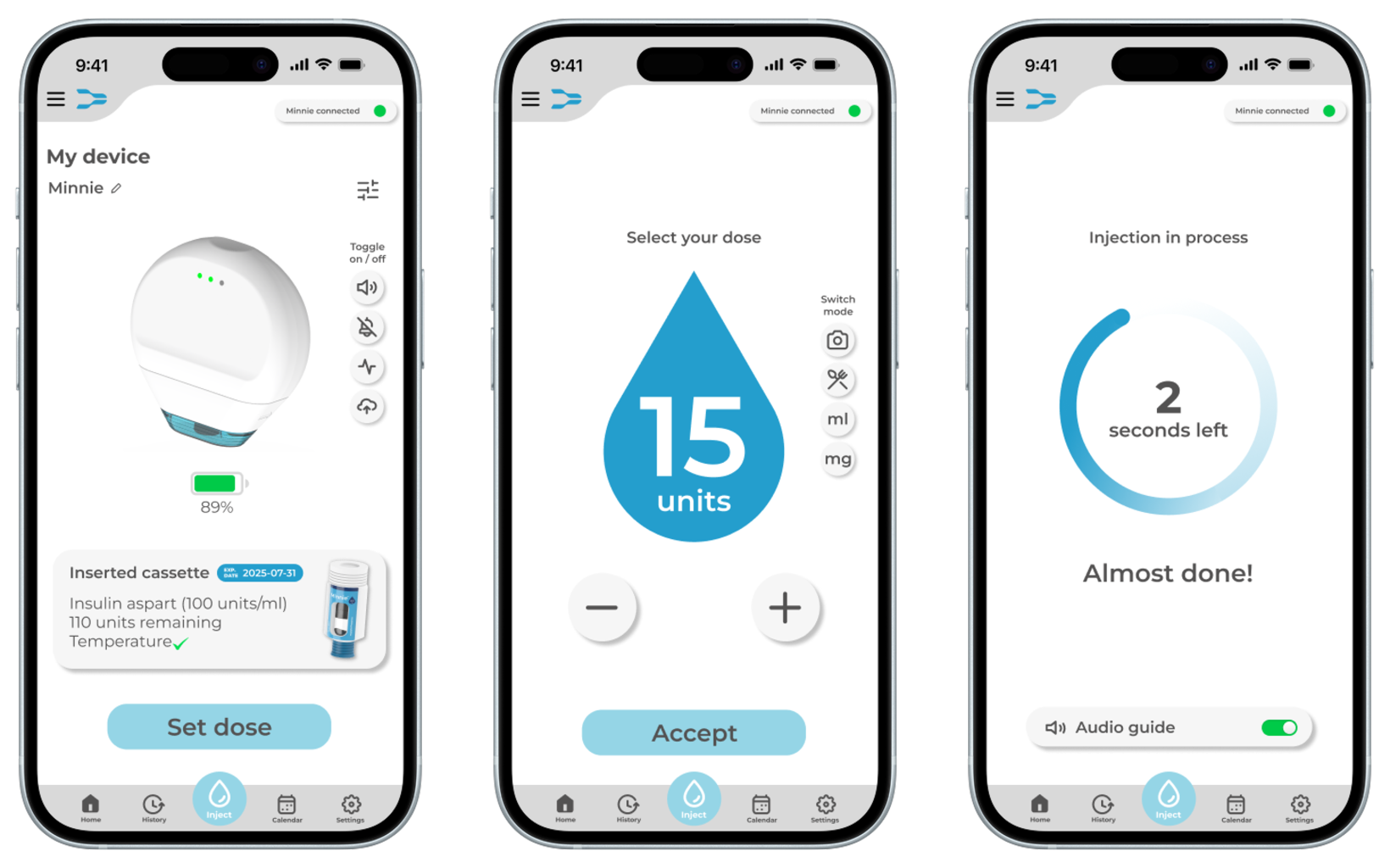
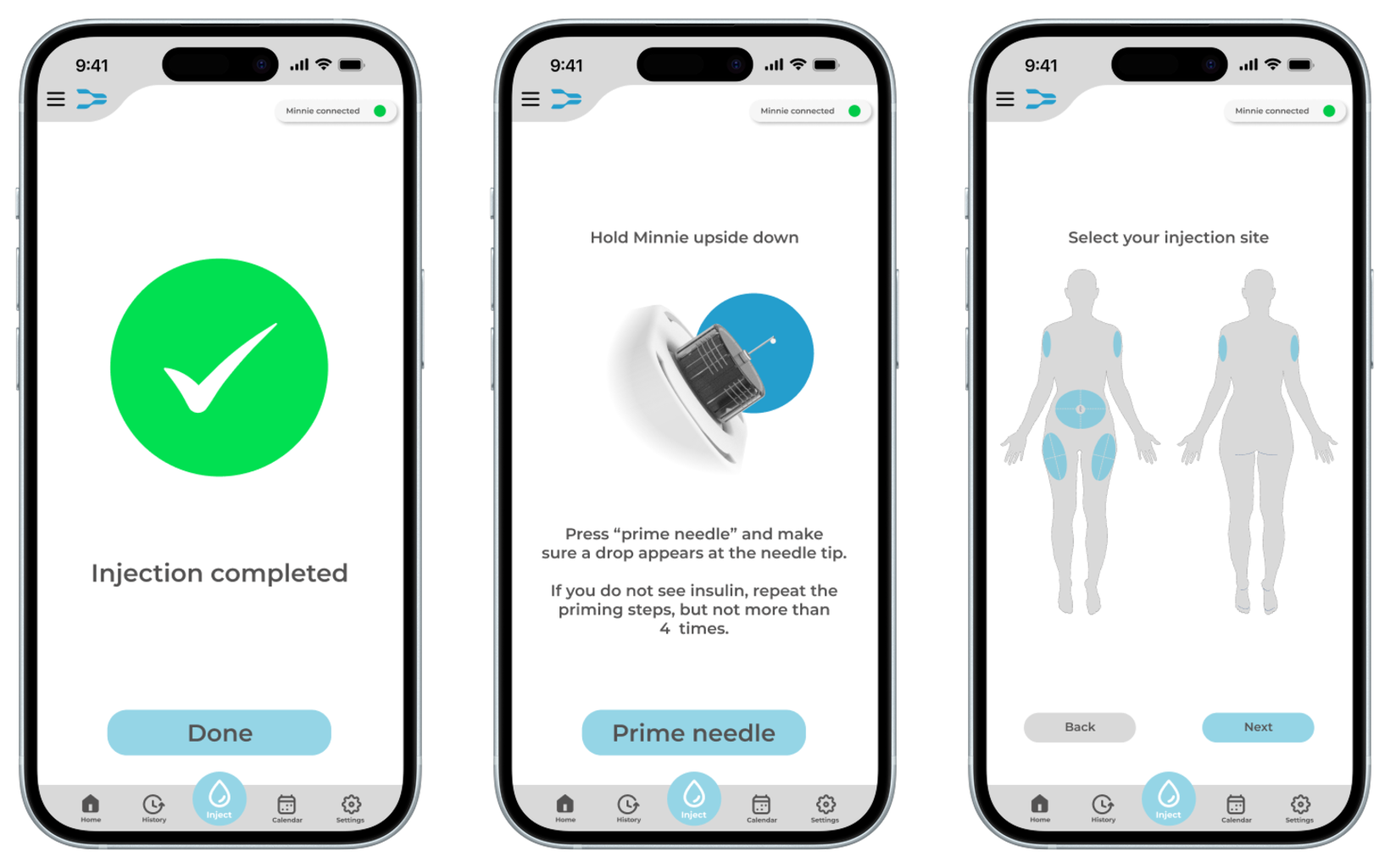
The app enhances the overall user experience and ensure safe administration. A first iteration of the app interface was created, incorporating the most important functions for users identified during the research phase. One of the key safety functions of the app is dose control as the injection device should not be capable of delivering a dose unless it has been confirmed through the app. Before an injection is initiated, the user must select and confirm the exact dose within the app. This ensures that even if the injection button on the device is pressed unintentionally, no medication will be administered.
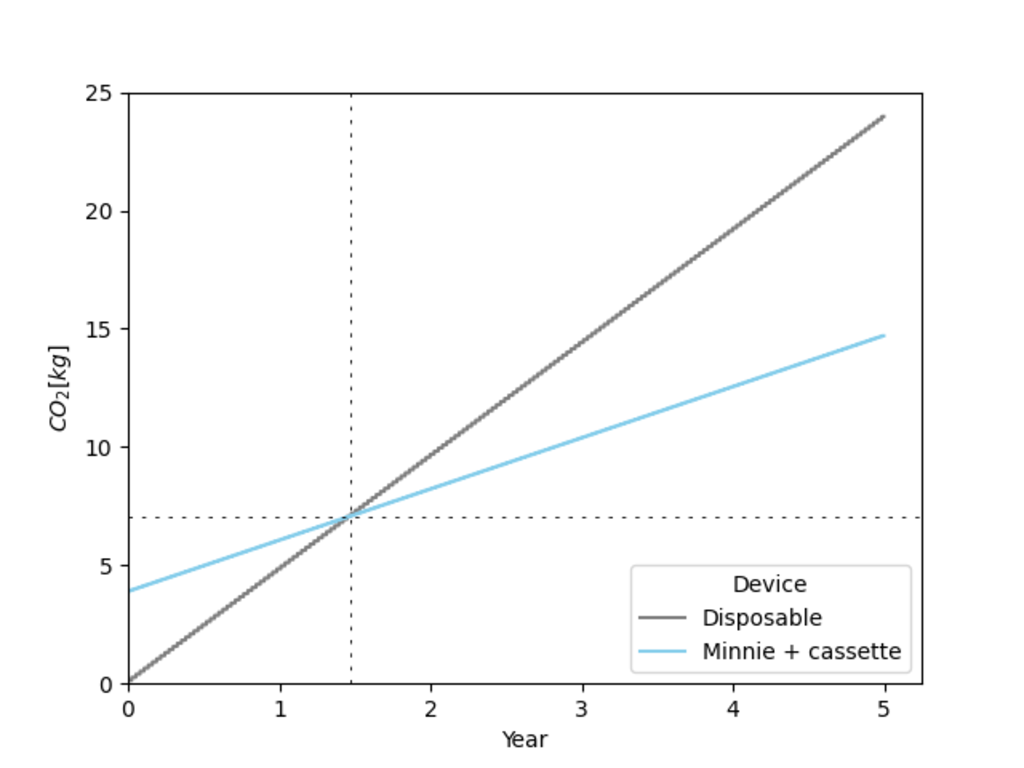
To make Minnie truly sustainable, the logistics surrounding proper disposal need to be taken into consideration. In order to avoid the worst case scenario: disposal in household waste, Minnie should be offered together with a substantial take-back program. The main goal of the take-back program is to collect empty cassettes, refill them and put them back on the market - ensuring a closed-loop system for these single-use components. Refilling a cassette entails an intermediate-level disinfection of the cassette body, cover, hatch and cap, and a reassembly. The cartridge (vial, plunger, crimp cap and septum) are never reused, instead they are recycled and a new pre-filled one is simply inserted into the cassette during reassembly. The reused cassettes are tested to ensure that safety and hygiene standards are fulfilled before being put back on the market. When a cassette has reached the end of its lifecycle (a minimum of 20 refills), it is properly and efficiently recycled by the manufacturer according to the waste hierarchy.
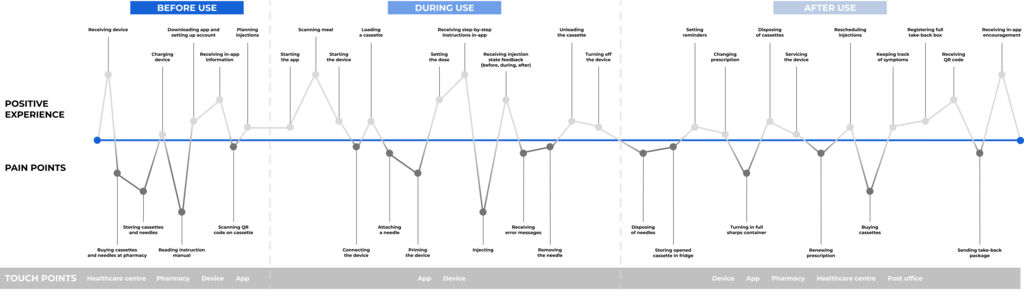
Pharmacies and post offices (including service points, postal agents and delivery companies) are central to the collection of used cassettes, and the main touch points for the service, as: 1) pharmacies are well-established centres for disposing of medical waste, and 2) post offices are a convenient and resource efficient way for delivery. A customer can choose whether to drop their cassettes off at the pharmacy as they use them, or they can opt to receive a collection box for the cassettes at the time of purchase. This collection box is then used to store the used cassettes until it is filled (around 10 cassettes), at which point it can be posted at the nearest post office by registering the return in the app and receiving an in-app QR code to show at the time of drop-off. Pick-up deliveries can be booked for customers with disabilities and other constraints (e.g. locational).
Centralising the disposal of used cassettes minimises the strain put on users while optimising the potential for circularity. Limiting the planning needed and the additional steps taken to return the cassettes are of utmost importance to make the take-back program work, as a complicated process could lead to customers choosing to throw used cassettes in the household waste for convenience. However, solely relying on convenience (e.g. pick-up delivery services) could lead to a higher environmental impact if it replaces more environmentally friendly routines and habits (e.g. walking and cycling). Therefore, it is important to balance this potentially negative effect when designing and implementing this kind of product service system.
To further align with the CE model, the take-back program should also accept broken devices or devices that are no longer required, e.g. when treatment is changed, paused or deemed unnecessary. These devices can be serviced and/or repaired and put back on the market, or be disassembled for spare parts and/or recycled depending on the state of the device and its components. In addition to pharmacies and post offices, healthcare centres should be added as a touch point for device collection.